News Articles
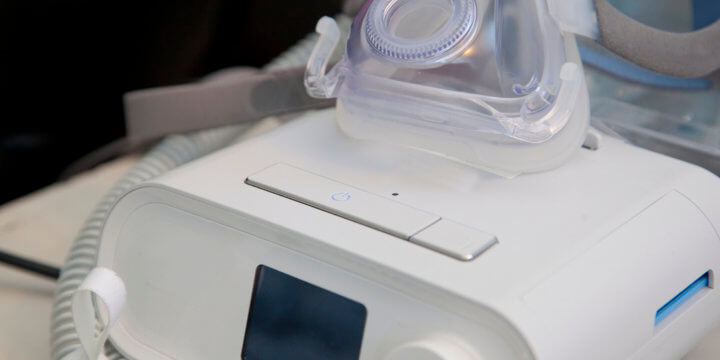
Philips CPAP Recall Update | Expanding Recall of Philips Machines
Since the Philips recall was first announced, it has been expanded several times to reflect a growing number of Philips Respironics ventilators, BiPAP, and CPAP machines, which present serious health and safety risks to users. The following devices have been recalled due to potential health risks:
- A-Series BiPAP A30
- A-Series BiPAP A40 (ventilator)
- A-Series BiPAP Hybrid A30
- A-Series BiPAP V30 Auto (ventilator)
- C-Series ASV (ventilator)
- C-Series S/T and AVAPS
- DreamStation
- DreamStation ASV
- DreamStation Go
- DreamStation ST, AVAPS
- Dorma 400
- Dorma 500
- E30
- Garbin Plus, Aeris, LifeVent (ventilator)
- OmniLab Advanced+
- REMstar SE Auto
- SystemOne ASV4
- SystemOne (Q-Series)
- Trilogy 100 (ventilator)
- Trilogy 200 (ventilator)
All of these machines are designed to be used while the patient is sleeping, so contain an insulating foam, which is supposed to reduce the noise associated with the machine. Unfortunately, the insulating foam can break down, exposing the user to toxic, carcinogenic fumes—a fact that was known to Philips, but hidden from the public, until recently.
Risks of Inhaling Toxic Foam
There are many potentially serious risks associated with prolonged use of a Philips machine. Indeed, a wide range of adverse events have already been reported to the FDA related to the breakdown of the foam in the machines.
Our team is already investigating claims on behalf of individuals that have experienced one (or more) of the following symptoms:
- Cancer, including cancers involving the respiratory tract, like palette cancer, esophageal cancer, and nasal cancer
- Pneumonia
- Asthma
- New difficulty breathing
- Other respiratory problems, like irritation nose or airway
- Infection
- Headache
- Dizziness
FDA Update: What to Do If You Use a Recalled Device
In November 2021, the FDA provided additional information regarding the recall. Click here to view the update.
You Can Ask your Doctor About Getting a Different Machine
If you use a recalled Philips Respironics BiPAP device, CPAP device, or ventilator, you can speak with your doctor about getting a machine made by a different company. Talk to a physician as soon as possible so that he or she can help determine the correct action for you to take. Your physician may advise you to stop using your recalled device, use another device that is not a part of the recall, use other treatments for sleep apnea such as positional therapy or oral appliances, or take another action depending on the device you use.
Watch for New Symptoms
Not everyone will experience symptoms right away. It is important to continue to monitor yourself for any new symptoms, like those listed above, which may be serious and could be caused by your use of a Philips CPAP, BiPAP, or ventilator machine.
Ben Martin Law Group Can Help
If you or a loved one has been suffering problems related to a Philips CPAP, BiPAP, or ventilator machine, call Ben Martin Law Group at (214) 761-6614, or contact us at MBinfo@martinbaughman.com, for a free, no obligation initial consultation. Our attorneys have helped thousands of victims of accidents and injuries caused by medical devices and pharmaceutical design defects. We take on global and multinational corporations every day and hold them accountable for their actions. Call today.